Excellence QBRI Insights: Factors Influencing COVID-19 Severity
The majority of COVID-19 cases are mild and patients recover quickly. However, about 15% of the cases are severe and require intensive care. This week, QBRI’s experts discuss various aspects that may contribute to COVID-19 progression.
COVID-19 and Comorbidities
The global COVID-19 mortality rate (3-7%) appears to be far higher than for seasonal flu (0.1%). The excess deaths caused by COVID-19 have been associated with age and other underlying health conditions, termed ‘comorbidities’, meaning the presence of more than one disease at the same time.
Age has been identified as the single most critical factor for COVID-19 severity and mortality. According to a recent study, the global case fatality rate was 1.4% under the age of 60, but in the age group above 60 it was 4.5%, and in those above 80 years it jumped to 13.4% (1). Other studies have shown that underlying health conditions such as hypertension, diabetes, heart diseases, obesity and cancer are major risk factors.
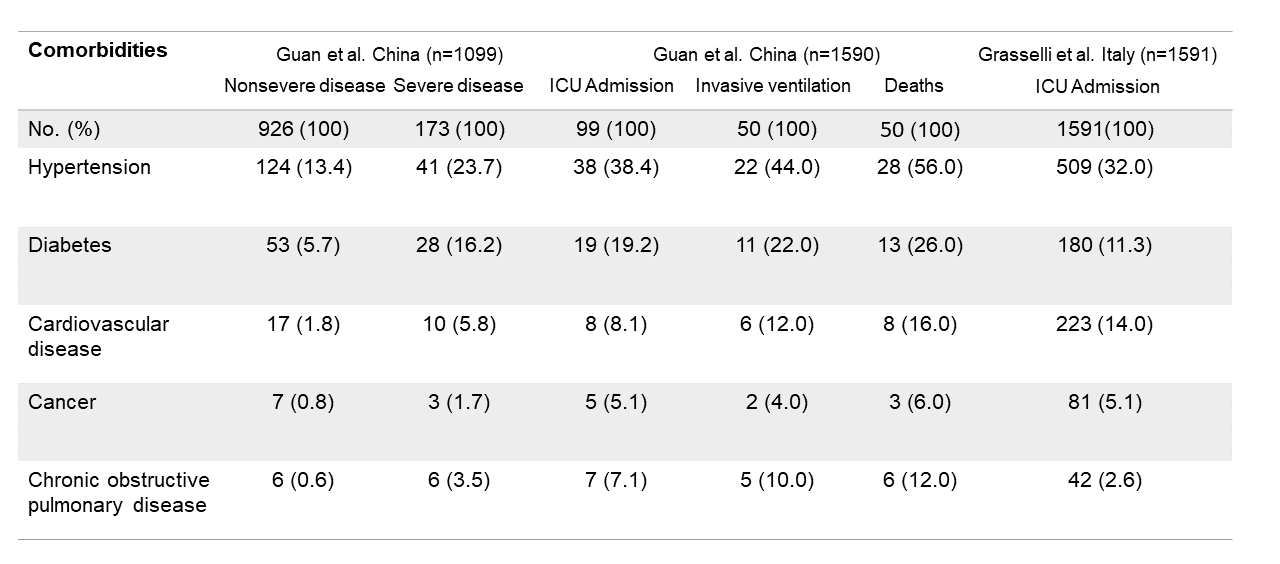
In a Chinese cohort study of 1,099 COVID-19 patients, there were more patients with comorbidities among severe cases compared to mild cases (38.7% vs. 21.0%), with hypertension being the most common comorbidity (2).
Another Chinese study of 1,590 hospitalized COVID-19 patients indicated that patients with any comorbidities had a higher probability of intensive care unit (ICU) admission (13.5% vs. 3.8%) and death (8.8% vs. 1.3%) than patients without comorbidities.
Moreover, probabilities of ICU admission, invasive ventilation, and death were higher in patients with two or more comorbidities compared to patients with a single comorbidity (3). Similar observations were made in a retrospective study in Italy including 1,591 ICU-admitted COVID-19 patients, revealing that 68% had at least a single comorbidity; and hypertension (32%) was the most common one (4). Other comorbidities included cardiovascular disease (14%), hypercholesterolemia (11.8%), diabetes (11.3%), cancer (5.1%), and chronic obstructive pulmonary disease (2.6%). Smoking has also been indicated as a risk for COVID-19 severity (5).
These observations beg the question, why do comorbidities increase the severity of COVID-19? A common feature in patients with longstanding comorbid conditions is a debilitated immune system (6). A weakened immune system may not be able to detect and eradicate a viral infection, or, alternatively, it can be overstimulated such that it targets even healthy tissue.
Cytokine storms in COVID-19 patients
Cytokines are small molecules secreted by certain cells of the immune system to regulate the body’s response against an infection (7).
To understand the cytokine storm, we need to understand what happens once the virus enters the body. Following invasion, the virus replicates inside the patient’s cells – cells in the lungs, for example – triggering an immune response that attracts more immune cells to the site of infection. This concerted phenomenon is termed inflammation.
While in some patients the level of inflammation can remain controlled, in others a hyper-activated immune response occurs, resulting in severe tissue damage and organ failure – a common cause of COVID-19 mortality (8, 9).
Cytokine storms are not specific to COVID-19, and have also been observed in Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) as well as non-viral diseases such as multiple sclerosis and pancreatitis (10). The reason why some COVID-19 patients develop cytokine storms and others do not is still poorly understood; however, it could partially be attributed to underlying comorbidities and genetics.
While several research groups are working towards developing treatments to counteract the cytokine storm, others are utilizing the cytokine profile to support diagnosis of COVID-19, and more importantly to determine the disease stage.
Several studies have documented the upregulation of pro-inflammatory cytokines in the blood, including interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and interferon gamma, especially in ICU-admitted COVID-19 patients (11, 12). A study in Guangzhou, China reported that the number of T cells, a crucial type of white blood cell fighting at the forefront of an infection, and the levels of IL-6 were significantly elevated in eight out of 11 COVID-19 patients with severe acute respiratory distress (13). A larger study from Wuhan showed a link between increased levels of IL-6 and the risk of death (14).
Many technologies are currently emerging to quantify the levels of COVID-19-related cytokines as potential biomarkers for the disease; important among those are interferon alpha, TNF- α and IL-6 (15). Measuring the levels of cytokines early during an acute infection will certainly influence our understanding of the cytokine storm onset, the course of disease progression and its impact on systemic damage. This may enable medical practitioners to identify cases that require increased vigilance or intensive care at an earlier stage.
Angiotensin converting enzyme 2 (ACE2): the ‘double-edged sword’ for COVID-19
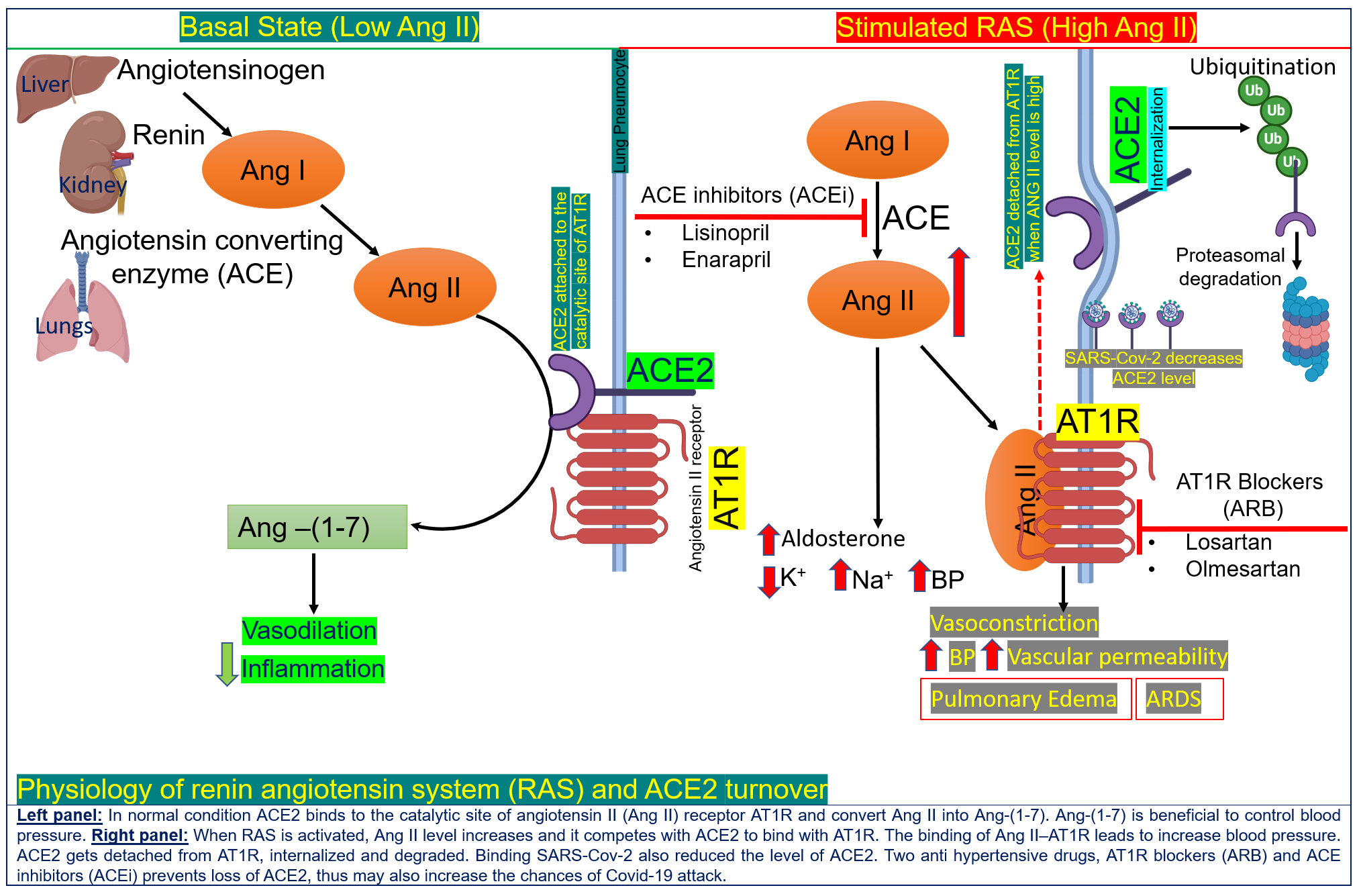
Apart from comorbidities and cytokine storms, the extent of viral entry and replication in human cells affects COVID-19 severity. SARS-CoV-2 hijacks an integral component (angiotensin-converting-enzyme 2, or ACE2 for short) of an important human hormone system (renin angiotensin system, RAS), which plays a crucial role in blood pressure regulation, metabolism, and inflammation (16).
ACE2, present on the surface of lung, kidney, intestine and blood vessel cells, has been identified as the receptor for the coronavirus and thus can be considered the ‘entry door’ for the virus into the cell. (17).
The level of ACE2 is substantially increased in patients with type 1 or type 2 diabetes. These diabetic patients are treated with drugs (ACE inhibitors, or ACEIs) that block the function of ACE and another important protein in the RAS system, angiotensin II type-I receptor (ARBs) (18).
ACE inhibitors and ARBs are also commonly used medications to treat hypertension and their use results in a further increase of ACE2 in cells (19). Hence, diabetic and hypertensive patients treated with ACE inhibitors and ARBs have very high levels of ACE2 and are at a potentially higher risk of developing severe and fatal COVID-19.
However, ACE2 receptors also exhibit biological functions that may be helpful in the context of COVID-19. A pivotal protective function of ACE2 is the reduction of angiotensin II (to other forms of angiotensin 1-7) (20), which has been associated with triggering a variety of adverse effects, including myocardial dysfunction, enhanced inflammation, obesity-associated hypertension and increased blood coagulation (17, 21).
Therefore, ACE2 is a ‘double-edged sword’ for COVID-19 and has become the topic of vigorous clinical debate as to whether COVID-19 patients with diabetes and hypertension should continue to be treated with ACE2-stimulating drugs. The results from recent retrospective studies on the association of ACEi/ARB and COVID-19 mortality are inconclusive as they included only a small number of patients (22).
It is currently advised that patients with cardiac diseases, hypertension or diabetes, who are treated with ACE2-increasing drugs, should be monitored with the utmost caution. To date, there is no evidence to suggest that another class of anti-hypertensive drugs, calcium channel blockers, increase ACE2 levels and therefore these drugs could be considered as an alternative treatment for some COVID-19 patients.
Section Contributors
COVID-19 and comorbidities: Dr. Vijay Gupta and Dr. Yoshie Kobayashi
Cytokine storms in COVID-19 patients: Dr. Nour Majbour
Angiotensin converting enzyme 2: Dr. Abu Saleh Md Moin
Illustration by: Dr. Abu Saleh Md Moin
Editors: Dr. Adviti Naik and Dr. Alexandra E. Butler
For references, please click here.




