Excellence QBRI Insights: SARS-CoV-2 Stability and Neutralization
Most of us have been frantically disinfecting surfaces in fear of transmission of the SARS-CoV-2 virus. But is the virus airborne too? And once inside the human body, can the virus be neutralized? This week, QBRI experts discuss recent studies highlighting the surface and aerosol stability of SARS-CoV-2 and scientific advances in battling the infection with neutralizing antibodies.
SARS-CoV-2 Stability on Surfaces and in Aerosols
To prevent the transmission of SARS-CoV-2, and hence contain the spread of COVID-19, robust public health and hygiene measures are essential. A study of the stability of SARS-CoV-2 on several surfaces published in the New England Journal of Medicine indicated that the virus was detected longer on plastic (72 hours) and stainless steel (48-72 hours) than on cardboard (24 hours) and copper (4 hours) (1).
Thus, the virus may spread by touching a contaminated surface or object, which serves to emphasize the importance of regularly disinfecting surfaces, washing hands frequently with soap and water, and making sure to avoid touching the face.
Nevertheless, the Centers for Disease Control and Prevention (CDC) released an updated report on May 11 that stated: “The virus does not spread easily via contaminated surfaces,” providing us with some relief from zealously wiping down groceries, doorknobs, keyboards, and other commonly touched surfaces.
Although the CDC still recommends to routinely clean and disinfect high-touch surfaces, the main mode of transmission of SARS-CoV-2 is thought to be close and prolonged contact with people, due to the high risk of inhaling respiratory droplets when an infected person coughs, sneezes, or even talks.
The aerosols generated by talking are small respiratory droplets less than 10 microns (one micron is one millionth of a meter) in diameter. While one report revealed that the virus lingered in aerosols for three hours (1), another experiment in which a speaker loudly repeated the phrase “stay healthy” for 25 seconds in a cubic 226-L enclosure, demonstrated that the aerosolized droplets lingered for 8-14 minutes (2).
Moreover, small droplets in a poorly ventilated room are reported to persist far longer than in a well ventilated room (3). Air samples taken from the rooms where COVID-19 patients were treated (between 5-18 days) showed on average an 80% probability of the presence of SARS-CoV-2 particles (4). The size and number of aerosol particles and their viral load may be additional factors that determine the risk of exposure.
These studies emphasize the current necessity to wear masks in public spaces, ventilate rooms, maintain a two-meter social distance, avoid crowds, and talk in open spaces.
Neutralizing Monoclonal Antibodies Against COVID-19
Even with nearly six million confirmed cases and 360,000 deaths from COVID-19 having occurred worldwide, sooner or later advances in science and healthcare will enable the discovery of the ultimate therapy that will protect humans from COVID-19. In this race to find an effective treatment, the quest for effective blocking or neutralizing antibodies (nAbs) is always the “must-do-first” response to any novel pandemic.
An antibody is a ‘Y-shaped’ molecule that is generated by the body’s immune system in response to a viral infection in order to neutralize the virus and thereby allow the body to defend itself against the infection. Once a selective antibody against the virus has been identified, these can be engineered and produced at a larger scale outside the body to generate uniform neutralizing antibodies, commonly used as a drug in the treatment of many disease types.
Within the body, SARS-CoV-2 attaches to the ACE2 receptors on the host cell surface through the receptor binding domain (RBD) of the spike protein S1 subunit (https://www.hbku.edu.qa/en/news/qbri-insights-biology) (5, 6). Therefore, blocking the ‘virus-host attachment’ using specific neutralizing antibodies against either host cell ACE2 receptors or the viral RBD in the spike protein would reduce viral penetration, which is the first critical step to protect against COVID-19.
Several modes of action are possible for nAbs in neutralizing SARS-CoV-2 (illustrated in Figure 1):
- Antibodies binding the RBD may sterically hinder binding to the ACE2 receptor, thereby preventing virus infection (7).
- nAbs may induce the aggregation of viral particles, which cause a reduction in penetration of virus into cells.
- In the post-attachment step, nAbs on the virus surface could dampen viral internalization, leading to viral degradation and clearance.
- nAbs could also block fusion of virus particles when they intercalate (or insert) between the virus and the cell membrane.
- Lastly, nAbs might bind to the virus surface, and block the replication of the virus even after internalization into cells (8-10).
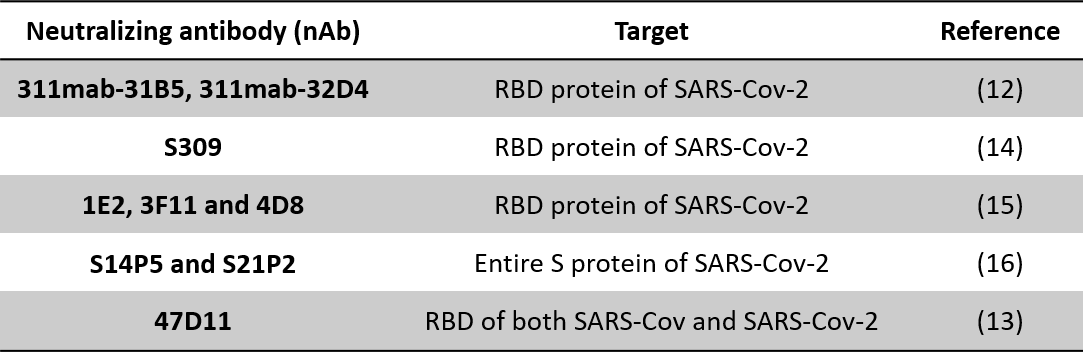
A number of the nAbs proposed against SARS-CoV could be useful against SARS-CoV-2 as well because of the similarity between the two viruses. Some of these nAbs neutralize the S1 fragment and block the interaction of the S1 subunit protein with the ACE2 receptor on the host cells; these include 80R, CR3014, CR3022, F26G18, F26G19, m396, and 201 (11). However, to date, only a few studies of specific nAbs against SARS-CoV-2 have been reported. 311mab-31B5 and 311mab-32D4 human nAbs could strongly and specifically bind the RBD protein.
These nAbs neutralize genetically-engineered virus (pseudovirus) entry into host cells with ACE2 receptors and hence could similarly efficiently block SARS-CoV-2 and ACE2 interaction (12). Very recently, a new human nAb (47D11) that binds the S1 of both SARS-CoV and SARS-CoV-2 viruses showed cross-neutralizing activity by a mechanism that is different from ACE2 receptor binding interference (13).
With enormous efforts and cutting-edge biochemical and molecular biology techniques being utilized to identify SARS-CoV-2 nAbs, it is only a matter of time before we see these being clinically implemented for COVID-19 treatment Some SARS-CoV-2-specific nAbs and their target sites are listed in Table 1.
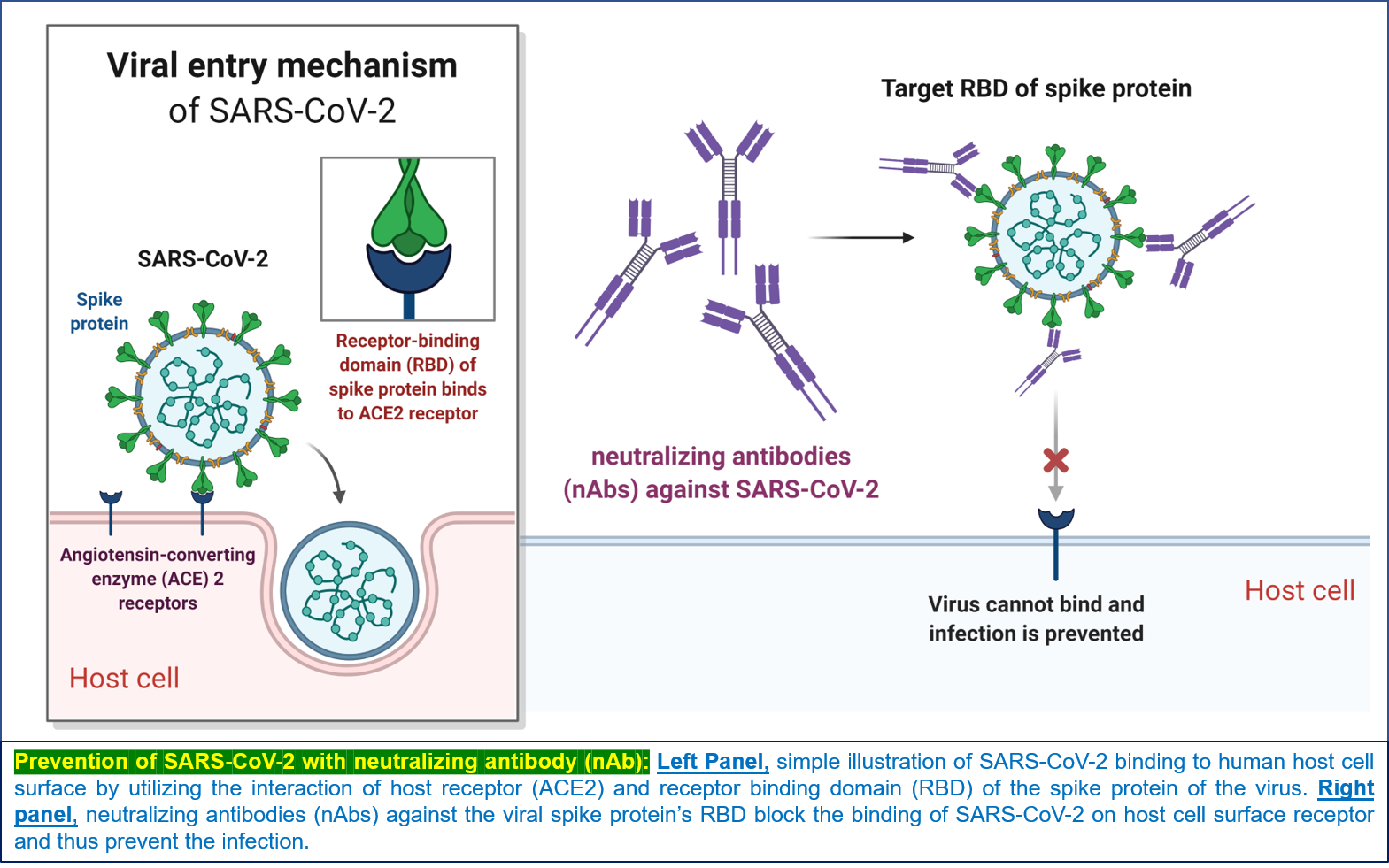
Figure 1: Prevention of SARS-CoV-2 infection with nAb
Table 1: List of neutralizing antibodies (nAbs) identified so far against SARS-CoV-2 spike (S1) protein receptor binding domain (RBD) (References (12-16))
Section Contributors
SARS-CoV-2 stability on surfaces and in aerosols: Dr. Yoshie Kobayashi (Postdoctoral Researcher, QBRI)
Neutralizing Monoclonal Antibodies (nAbs) against COVID-19: Dr. Abu Saleh Md Moin (Postdoctoral Researcher, QBRI)
Illustration by: Dr. Abu Saleh Md Moin
Arabic text validation: Dr. Nour K. Majbour
Editors: Dr. Adviti Naik (Postdoctoral Researcher, QBRI) and Dr. Alexandra E. Butler (Principal Investigator, QBRI)
For references, please click here.




