
Since the start of the COVID-19 pandemic, global research efforts have been directed to improving our understanding of the disease, its progression, and opportunities for intervention to treat patients. This week, QBRI experts discuss various parameters that can reflect the COVID-19 patient status and highlight key results from early clinical trials investigating the efficacy of repurposing anti-viral drugs for COVID-19 treatment.
The Role of Biomarkers in Diagnosing COVID-19
Patients infected with COVID-19 are arriving at hospitals with different clinical symptoms. Fever, cough, dyspnea (shortness of breath), and acute respiratory distress syndrome are among the most common (1). However, the clinical symptoms of COVID-19 are not disease-specific, making the conventional clinical criteria suboptimal for assessing disease progression.
The gold standard for diagnosis remains the Polymerase Chain Reaction (PCR) molecular test https://www.hbku.edu.qa/en/news/qbri-insights-biology, and the limitations surrounding the clinical criteria could be overcome by the implementation of non-PCR diagnostic biological markers - biomarkers.
Biomarkers are objective measures of a disease state, disease progression and/or susceptibility to a disease. Biomarkers could monitor COVID-19 progression, reflect disease state (i.e. mild, severe, or critical) and deepen our understanding of underlying disease mechanisms. In addition, these biomarkers may be advantageous in early diagnosis of COVID-19 in asymptomatic patients who do not present with any obvious clinical symptoms.
Reliable biomarkers could also assess the patient response to treatment in the numerous clinical trials currently being conducted for drugs to treat COVID-19. Among the most prominent biomarkers explored for COVID-19 are white blood cell count (2), platelet count (2), C-reactive protein (CRP) (3), cardiac troponin (3), interleukin-6 (IL-6) (4), renal biomarkers (5), and angiotensin-converting enzyme (ACE) polymorphisms.
Since white blood cell count is influenced by many other factors, it does not provide reliable and specific insight about the COVID-19 patient state (2). Platelet count is a simple, economical, and rapid laboratory test that can help discriminate between COVID-19 patients with and without severe disease. Several studies have shown that sicker COVID-19 patients have lower platelet counts (2).
CRP is a non-specific marker of inflammation. Its levels are significantly elevated in severe COVID-19 cases. It is also one of the first markers to change in response to infection with SARS-CoV-2 (3).
IL-6 is another marker of inflammation that sharply rises in severe COVID-19 cases. Serum levels of IL-6 in COVID-19 patients with complications were several times higher compared to those with mild cases.
As mortality rates are high among COVID-19 patients with cardiovascular conditions, cardiac troponin has the potential to serve as a marker of disease state and mortality. The elevated levels of serum cardiac troponin are an indicator that the patient requires intensive care and close monitoring (3).
Similarly, patients with chronic kidney disease are at higher risk of severe forms of COVID-19 (5). The elevation of renal function biomarkers is also associated with increased severity of COVID-19 and the subsequent need for ventilation support and intensive care.
Genetic variants in ACE https://www.hbku.edu.qa/en/news/influencing-covid-19-severity), the gateway for SARS-CoV-2 infection into human cells, is another factor potentially determining the variable prevalence of COVID-19 around the different continents.
Taken altogether, a combination of several biomarkers reflecting different trajectories related to COVID-19 could potentially provide insights about the disease severity, prognosis, and response to the treatment.
Anti-viral Drug Strategies for COVID-19
The COVID-19 pandemic warrants the urgent development of potential drugs to treat patients and protect high-risk individuals. There are currently no specific drugs and/or vaccines available for SARS-CoV-2. Numerous approved drugs and a few new drugs are reported to have anti-SARS-CoV-2 activity, but are still in the early stages of investigation (6).
Therefore, deciding the potential therapeutic regimens to treat COVID-19 patients still remains a major clinical challenge. Since the development of new drugs is a lengthy process, drug repurposing is an alternative emerging strategy (Table 1).
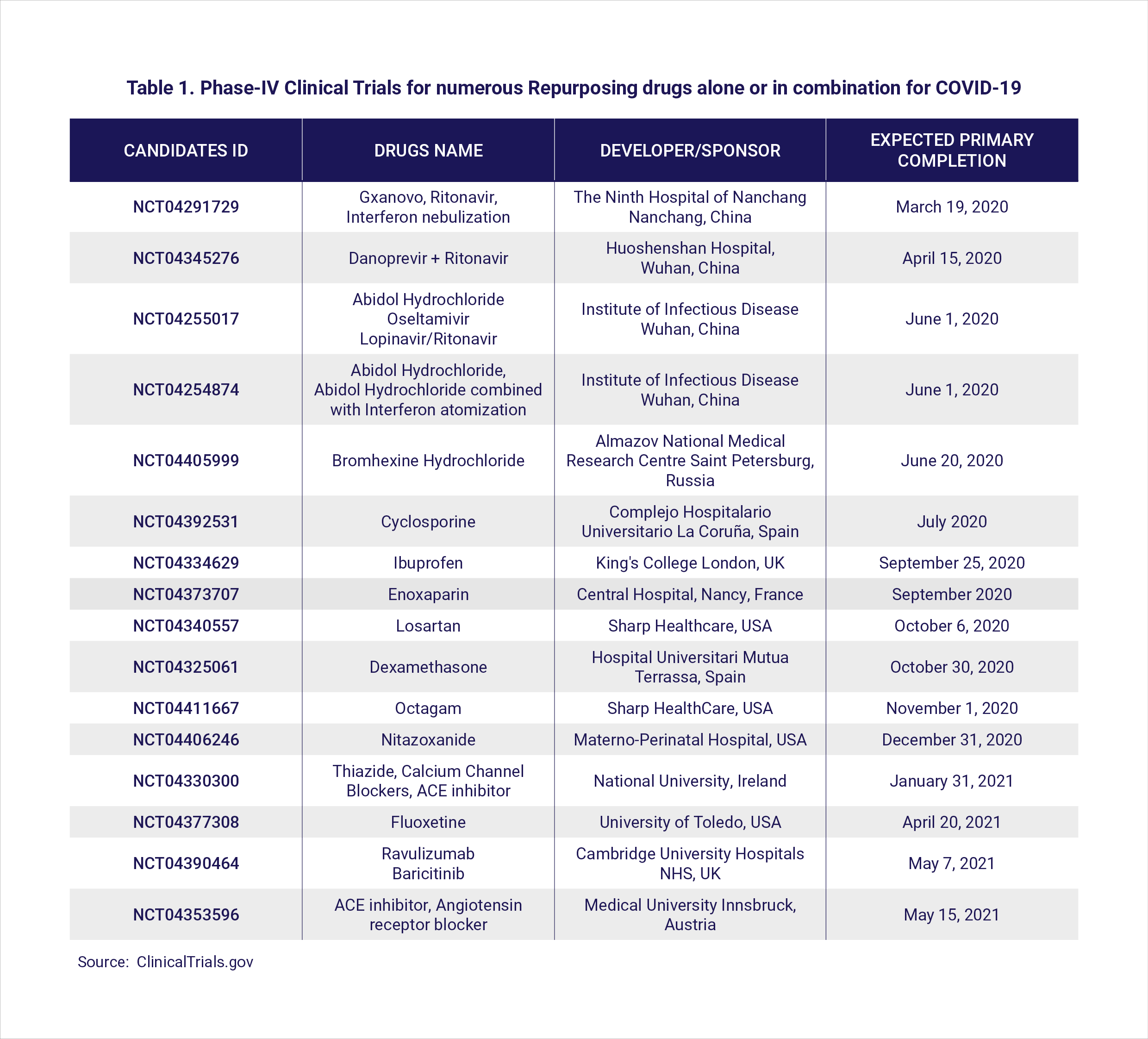
Anti-virals (drug names with a ‘vir’) are a broad class of drugs that reduce the ability of the virus to multiply (link to https://www.hbku.edu.qa/en/news/qbri-insights-biology). Many repurposed anti-viral drugs including remdesivir, favipiravir, lopinavir-ritonavir (anti-HIV drugs) as well as ribavirin and interferons (which activate the immune response against viruses), were found to be effective against beta-coronaviruses in preclinical studies (7).
In a recent study published in the Lancet journal, researchers from Wuhan, the Chinese city where the first cases of COVID-19 were reported, claimed that although administration of remdesivir had no clinical benefits for severe COVID-19 patients, it slightly reduced the recovery time (8).
This observation was confirmed in a larger study with 1,063 COVID-19 cases, whereby remdesivir treatment shortened the median time to recovery (15 days vs. 11 days) compared to the placebo control group (9).
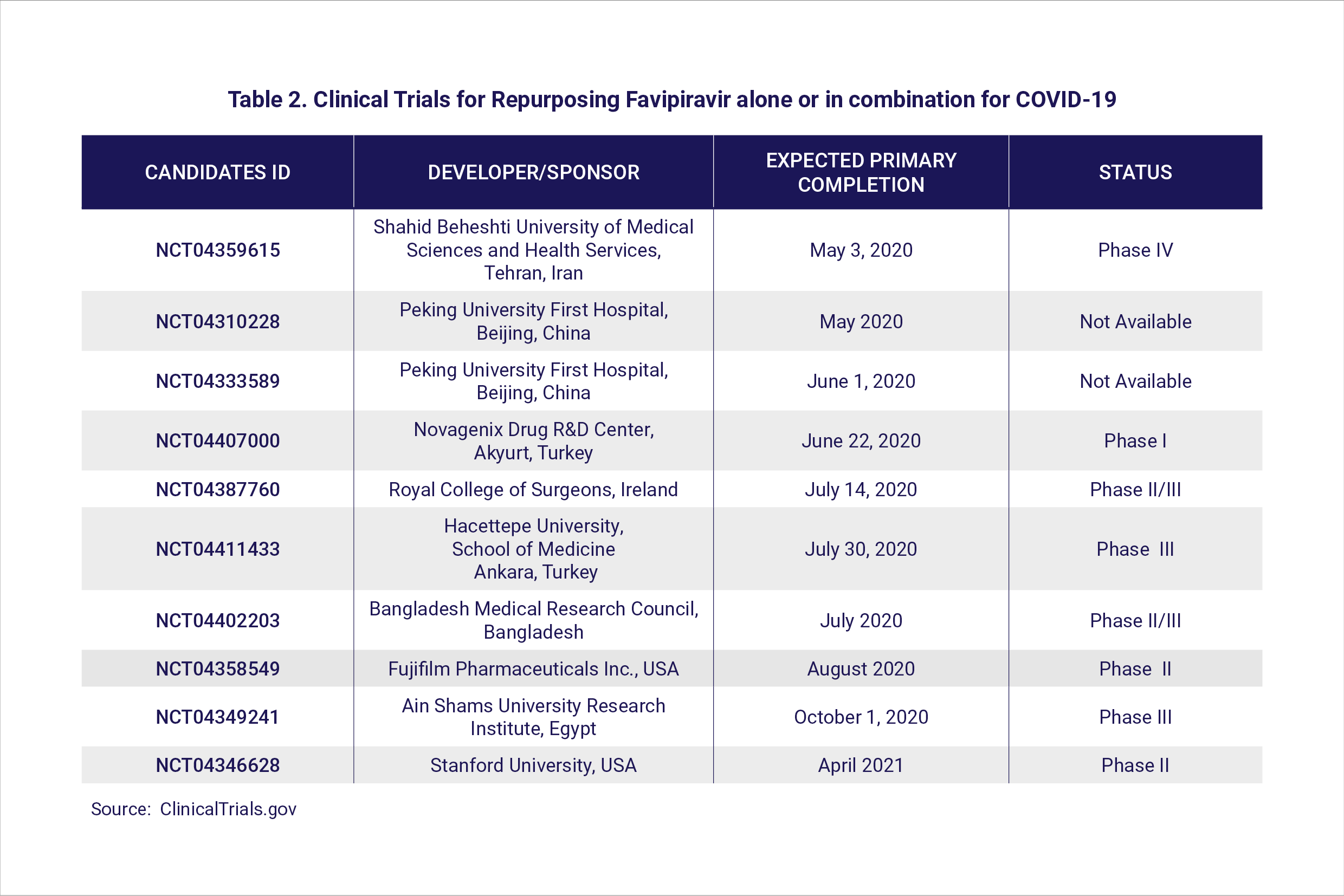
Avifavir (favipiravir) is a well-established and approved drug against influenza. At least 20 ongoing clinical trials are investigating the use of favipiravir for COVID-19 in different countries (Table 2). Favipiravir was found to be effective in reducing the time to recovery in two clinical trials in Wuhan. In Russia, preliminary results communicated by ChemRar Pharma also suggest that favipiravir administered to COVID-19 patients resulted in a shorter recovery period.
Earlier studies demonstrated that a combination of remdesivir/interferon-β1b with lopinavir-ritonavir offers better clinical effects against SARS-CoV-1 and Middle East Respiratory Syndrome (MERS)-CoV (10, 11). A multi-center, randomized phase-2 clinical trial (NCT04276688) in Hong Kong investigated a similar drug cocktail in 127 COVID-19 patients: 86 with the drug combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin and 41 with a single drug (lopinavir-ritonavir). The triple drug combination rapidly suppressed the viral load, reduced the time to recovery, improved clinical parameters, and reduced the risk of infection to the health-care workers (12).
Taken together, recent positive signs from repurposing anti-viral drugs have shed light on their potential efficacy against SARS-CoV-2. These observations now remain to be confirmed in larger clinical trials.
Section Contributors
The Role of Biomarkers in Diagnosing COVID-19: Dr. Nour K. Majbour (Senior Research Associate, QBRI)
Anti-viral Drug Strategies for COVID-19: Dr. Ramesh Elango (Postdoctoral Researcher, QBRI)
Arabic text validation: Dr. Nour K. Majbour
Editors: Dr. Adviti Naik (Postdoctoral Researcher, QBRI) and Dr. Alexandra E. Butler (Principal Investigator, QBRI)









