QBRI Insights: The Biology of SARS-CoV-2

Viruses, including SARS-CoV-2, the virus strain that causes the coronavirus (COVID-19), cannot thrive on their own and rely on infecting host cells in humans or animals to reproduce. Research in molecular biology has allowed us to explore the ‘inside information’ on SARS-CoV-2 and its pathogenic mechanisms. This week QBRI experts explain what happens once the virus finds a way to enter the human body and how this understanding is being implemented to design treatment interventions.
Life cycle of SARS-CoV-2
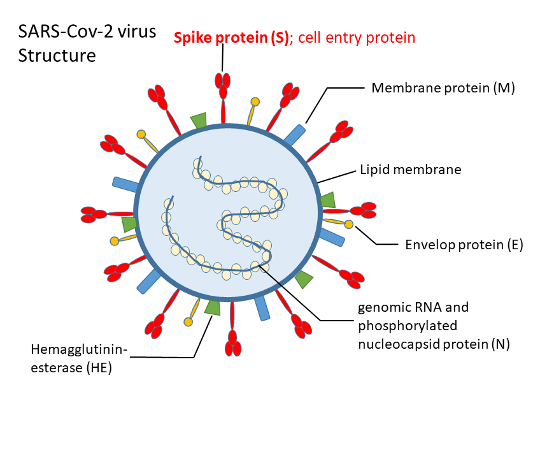
SARS-CoV-2 belongs to the beta-CoVs (betacoronavirus) family of viruses (1), similar to SARS-CoV, being ‘enveloped’ with an outer wrapping and housing a single strand of genomic ribonucleic acid (RNA) (2, 3). Genomic RNA of SARS-CoV-2 encodes the viral proteins such as a non-structural ‘replicase polyprotein’ with a function in virus replication; and structural proteins, including the spike (S), envelope (E), membrane (M), and nucleocapsid (N)- all of which assemble together to form the virus particle.
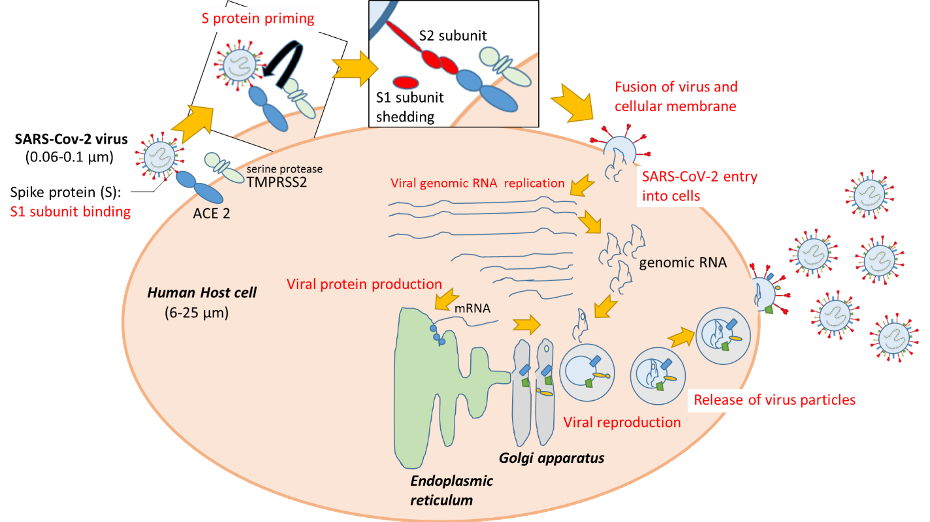
The life of a virus comprises: 1) infection - virus entry into host cells; 2) replication – virus reproduction using host cell resources; and 3) release of virus particles from host cells.
Once inside the body, SARS-CoV-2 has to enter the human cells to survive and replicate. Entry involves interaction of the virus spike protein (S) with a receptor protein on the host cell surface. Angiotensin-converting enzyme 2 (ACE2) has been reported as being the host receptor protein for SARS-CoV-2 entry, similar to SARS-CoV (4). ACE2, expressed in lung, heart, kidney, intestine, blood vessel and testis cells, is involved in the regulation of blood pressure and its malfunction has been associated with cardiovascular diseases (5-7). A recent study published in The EMBO Journal indicated trends of increased ACE2 expression with age, especially in males, in respiratory tract cells, possibly explaining the severity of the disease in the elderly (8).
The viral spike protein has two parts, S1 and S2. At first, the surface S1 part binds to host cell ACE2, and then a type of ‘molecular scissor’ on the host cell (serine protease TMPRSS2) cleaves the S protein called S protein priming (4).
Finally, the S2 part enables fusion of the SARS-CoV-2 and host cell, leading to SARS-CoV-2 entry into the human cell. Once inside, SARS-CoV-2 hijacks the host cell machinery to replicate its own RNA and manufacture or ‘translate’ its proteins. The viral genomic RNA and proteins assemble inside packages termed ‘vesicles’, and the newly formed SARS-CoV-2 particles are released from host cells.
Our immune system surveillance detects a virus infection and readily responds to eliminate the intruder. For instance, receptor proteins on host cells, such as toll-like receptors (TLRs), can recognize both the full virus particle or parts of it (9). Activation of the TLR promotes the production of pro-inflammatory cytokines and interferons (IFNs)- our body’s natural defense against invading viruses (10). Various immune cells sequentially assemble at the infection site and engage in a battle with viruses.
Consequentially, lung cells increasingly secrete mucus which impairs the normal functioning of the lung cells and exchange of gases (O2 and CO2), leading to infected patients coughing up sputum containing the virus.
The SARS-CoV-2 infection manifests as an upper respiratory inflammation, pneumonia, and severe acute respiratory syndrome (SARS). SARS is a pulmonary inflammation induced by excessive activation of the immune system, leading to long-term lung damage. In the most severe cases, multiple organ failure can ensue due to systemic inflammation, possibly leading to death.
Detection of the SARS-CoV-2 genome with RT-PCR
The current gold-standard diagnostic test for COVID-19, reverse transcription-polymerase chain reaction (RT-PCR), relies on detecting unique regions in the SARS-CoV-2 genome (11). In January 2020, the World Health Organization (WHO) published the first protocol with three RT-PCR tests designed to detect three different genes of the virus: 1) the first line of screening is for the E gene, (present in the “E” region that encodes the envelope protein) 2) a confirmatory test for the RdRp gene (in the “ORF1ab” region that encodes well-conserved non-structural proteins), and 3) an additional confirmatory test for the N gene (in the “N” region that encodes the Nucleocapsid protein) (12, 13).
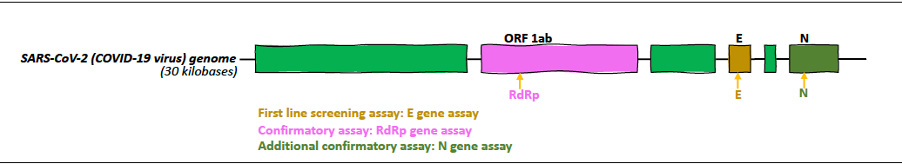
Diagnostic samples are of respiratory origin: nasopharyngeal swab or sputum. Once the sample is taken, it is first lysed using a nucleic acid extraction kit to release the SARS-CoV-2 RNA. Next, the extracted RNA is converted to cDNA (cDNA: complementary DNA), a quantifiable molecule, using an enzyme called reverse transcriptase or retrotranscriptase (hence the name "RT").
If present, the SARS-CoV-2 cDNA is amplified (millions of copies) by the polymerase chain reaction (PCR) and detected by a fluorescent chemical reaction. The real-time PCR detection system allows the sample to be quantified and to know how many copies of the virus are present per sample. Finally, if the reaction is positive, it shows a fluorescent signal above a predetermined threshold. However, if negative, there is just background noise or no fluorescence.
There is a latency period in which it is not yet possible to detect SARS-CoV-2 viral load in early stages of the infection. During an infection, the virus actively multiplies and viral RNA levels are at their highest when COVID-19 symptoms first appear, allowing accurate detection (14).
However, RT-PCR cannot detect a recovered COVID-19 case, as these can only be identified from antibody levels, and thus RT-PCR needs to be complemented by serological diagnostic methods that detect antibodies to SARS-CoV-2.
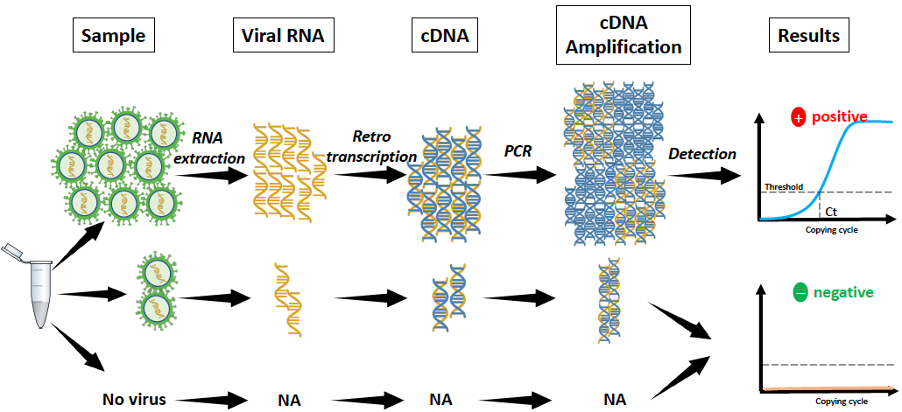
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
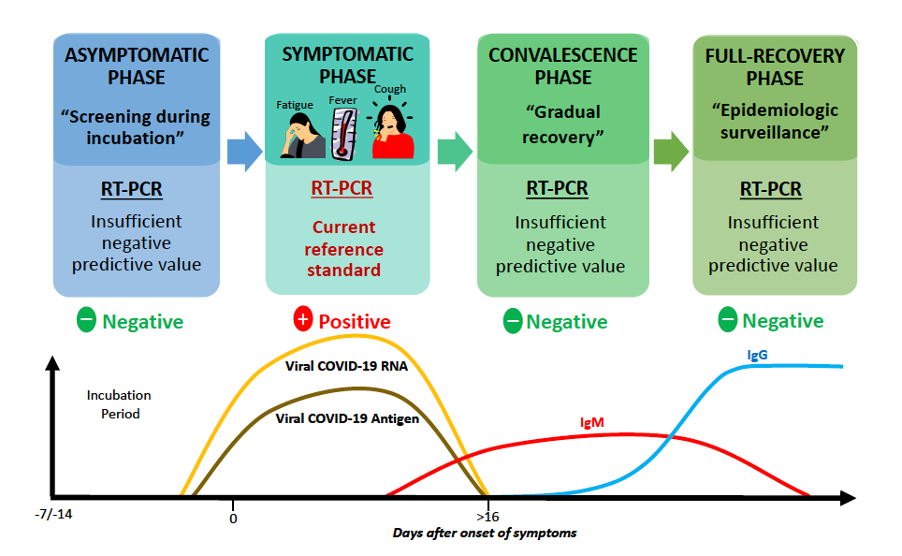
Detection of SARS-CoV-2 Infection
Drug repurposing: the first line of defense against the COVID-19 pandemic
A vaccine against COVID-19 would slow its spread around the world, ensure that fewer people would get symptomatic illness, and thus more lives could be saved. But the bitter truth is that even when researchers find a vaccine that works against the new coronavirus, it could be 12 to 18 months at best before it is ready for the public.
Even this time frame is a fraction of the usual time for vaccine development. As an emergency response to COVID-19, clinicians around the world are depending on ‘Drug Repurposing’ - an emerging strategy where existing medicines, having already been tested safe in humans, are redeployed to combat difficult-to-treat diseases.
Broad-spectrum antiviral agents (BSAAs) that have been deemed ‘safe-in-man’ through testing in early phase clinical trials have been touted as good drug repurposing candidates (15). Our understanding of the viral lifecycle steps provide potential targets for drug therapy (16).
Promising drug targets include nonstructural proteins (e.g., 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase), which share homology with other novel coronaviruses (nCoVs) (17).
Additional drug targets include viral entry and immune regulation pathways. In addition to reprofiling the existing drugs, the quest for de novo drugs against COVID-19 is ongoing. On April 9, 2020, scientists in Shanghai, China reported in Nature that a virtual high-throughput drug screening of over 10,000 compounds identified six new candidate drugs that inhibited COVID-19 virus’ main protease (Mpro), which is involved in virus replication (18). So there are positive signs regarding a new effective anti-viral drug against SARS-CoV-2.
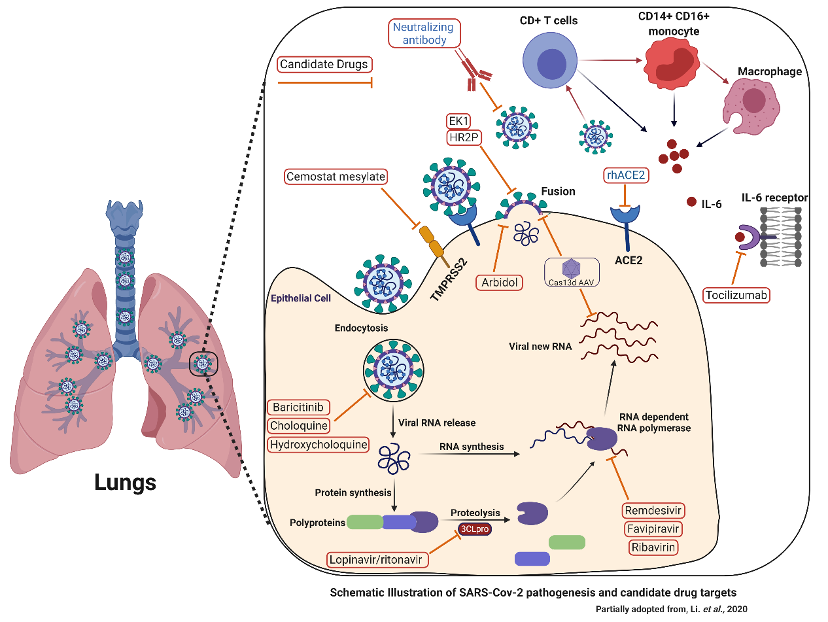
Candidate Drugs to Target SARS-CoV-2 Infection
Section Contributors:
Epidemiology: Dr. Yoshie Kobayashi and Dr. Vijay Gupta
Diagnostics: Dr. Salam Salloum-Asfar
Treatment: Dr. Abu Saleh Md Moin
Illustrations by: Dr. Yoshie Kobayashi, Dr. Salam Salloum-Asfar, Dr. Abu Saleh Md Moin
Editors: Dr. Adviti Naik and Dr. Alexandra E. Butler
For references, please click here.
Related News

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention