QBRI Insights: An Overview of the COVID-19 Pandemic
Coronaviruses are not alien to us. Human coronaviruses were first discovered in the 1960s and many different strains are known to date. So, what is unique about COVID-19? With the dizzying plethora of media publications available, one cannot help but feel overwhelmed. To address this issue, biomedical researchers at the Qatar Biomedical Research Institute (QBRI), part of Hamad Bin Khalifa University (HBKU), have set out to filter misinformation and provide the wider community with credible scientific information and developments relating to the disease. This week, QBRI’s experts provide an overview of COVID-19 and explore the current status of potential treatments.

The most trending topic in the news, social media, and our daily conversations is COVID-19, an acronym for COronaVIrus Disease of 2019, which is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Pandemics such as COVID-19 are not new to us. Viral pandemics have afflicted humanity throughout history, documented as early as the year 165-180 AD when the Antonine Plague occurred. Some recent viral outbreaks include swine flu (H1N1), Ebola, Severe Acute Respiratory Syndrome (SARS), and Middle East Respiratory Syndrome (MERS).
Epidemiology
Coronaviruses are a large family of RNA viruses that are the source of multiple illnesses, ranging from the common cold to more severe diseases such as MERS and SARS. Coronaviruses get their name from the Latin word “corona”, meaning crown or halo since under an electron microscope the virus structure looks like a solid body surrounded by bulbous surface projections of spike proteins like the solar corona. Coronaviruses are zoonotic in origin, meaning they are transmitted between animals and humans. SARS-CoV-2 has 80 percent genetic similarity to SARS-CoV-1 and 50 percent to MERS-CoV (1, 2).
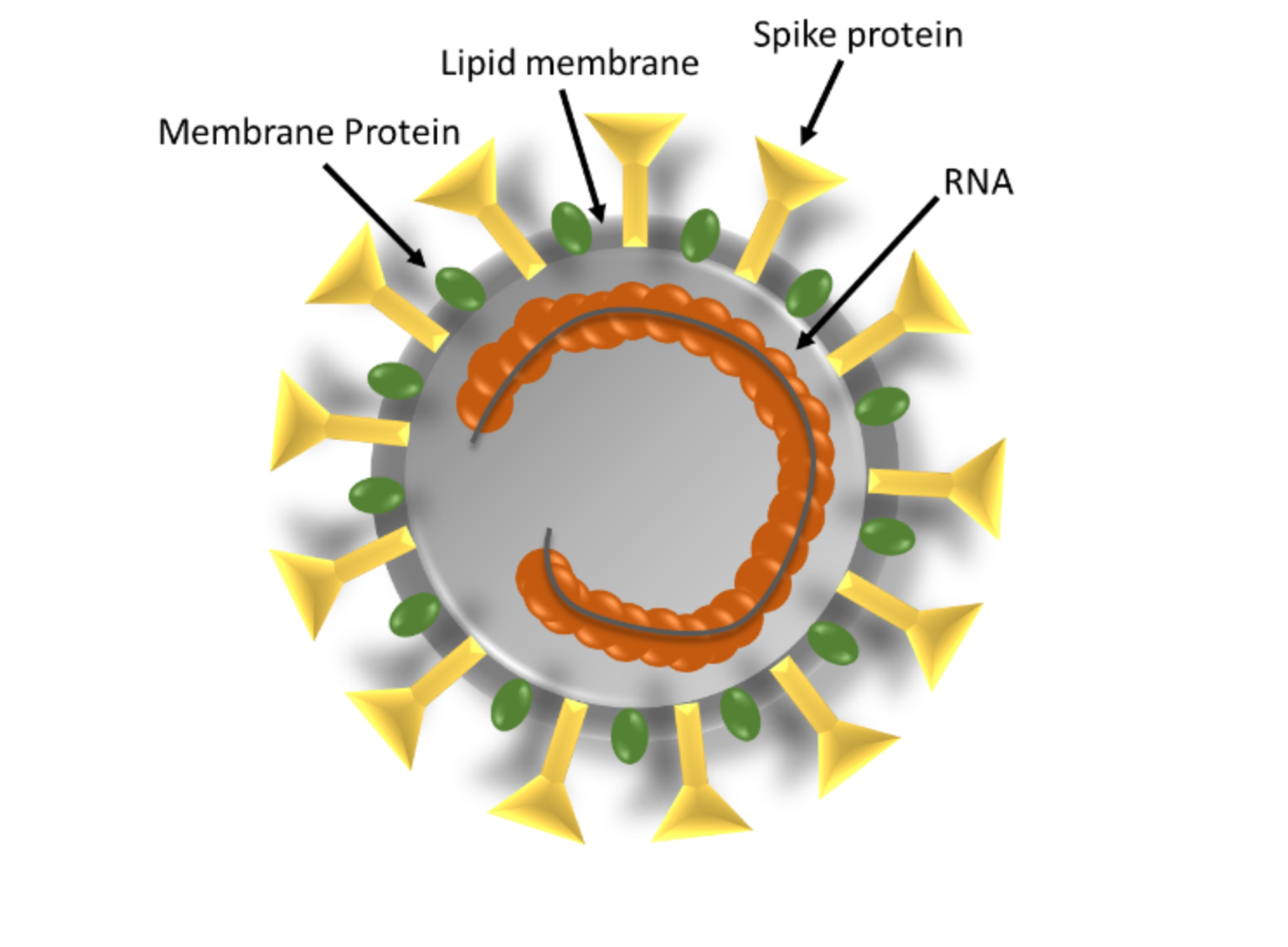
Schematic Structure of SARS-CoV-2
In mid-December 2019, the first case of COVID-19 was reported in Wuhan, China, apparently originating in a seafood and animal market (3). Subsequently, COVID-19 spread exponentially within China and to the rest of the world with 2,000,951 confirmed cases and 126,782 deaths related to COVID-19 in 210 countries; this includes 3,428 confirmed cases and seven deaths in Qatar, as per the data available on April 15, 2020 (4). Guan et al. (5) reported that, in a Chinese cohort study, only 1.9 percent of 1,099 COVID-19 patients had a history of direct contact with wildlife such as bats, while the majority of cases likely resulted from human-to-human transmission through direct contact or respiratory droplets spread by the coughing or sneezing of an infected individual. SARS-CoV-2 can also be detected in the gastrointestinal tract (feces), saliva, and urine, and therefore further investigations are required to elucidate these potential transmission routes (6).
Common symptoms of COVID-19 include fever, coughing, breathing difficulties, and loss of taste. However, in more severe cases, COVID-19 can lead to pneumonia, severe acute respiratory syndrome, multiple organ failure and even death. According to the latest World Health Organization (WHO) estimates, infected humans can show symptoms i.e. signs of infection, between one to 14 days of exposure, although most signs are apparent between five to six days. Moreover, a large number of patients do not show any symptoms (asymptomatic carriers) even though they carry the virus and can infect other people (7). According to the Chinese Center for Disease Control (CDC), the overall case-fatality rate (CFR) is higher with increasing age (highest fatality in >80 years age group) and in those with preexisting health conditions such as cardiovascular disease, diabetes, obesity, chronic respiratory disease, and hypertension (8, 9).
Current options for COVID-19 diagnosis
Since the first diagnosis of COVID-19 in December 2019, the phrase "tested positive" has been commonly used, with the WHO requesting countries worldwide to increase their testing capacity (10). The ability to quickly detect those infected with the SARS-CoV-2 is crucial for two reasons. First, to get a true picture of the pandemic, since the number of confirmed cases greatly underestimates the number of those really infected. The second reason is a consequence of the first; to understand the modes of transmission and take necessary actions to break the chain of infection, especially in the case of asymptomatic and undiagnosed infected people, who may unconsciously transmit the virus to others (10-13).
Clinicians determine if a patient should be tested for COVID-19 based on common clinical presentations such as fever, cough, and fatigue (14, 15). Depending on national protocols, they may consider local epidemiology: whether a patient has been in close contact with someone diagnosed with COVID-19 or has a history of recent travel from an area with sustained transmission of COVID-19. To diagnose COVID-19, a health specialist takes samples that mainly include a nasal and throat swab but may also collect additional specimens from saliva and blood. Transportation from the clinic to the laboratory for analysis must be quick and refrigeration is necessary to preserve the sample.
The majority of countries have expanded their capacity to diagnose COVID-19. To date, according to the Centers for Disease Control and Prevention (CDC) laboratory testing recommendations and guidelines, two types of testing are available, as described below (16).
Polymerase chain reaction with real-time reverse transcription (RT-PCR)
This is the frontline gold standard test for diagnosing the presence of SARS-CoV-2. The test is based on a series of chemical reactions that detect the presence of the viral genetic material. It is a common laboratory procedure and offers reliable results, provided the sample is collected correctly. This test has good sensitivity (i.e. the probability that a person with COVID-19 has a positive test result) and specificity (i.e. the probability that a person without COVID-19 has a negative test result). However, it is used to detect very recent or active infections (high viral load) and can only be carried out in laboratories equipped with specialized equipment (17).
Rapid diagnostic tests
Rapid test kits appear to be the better option for wider screening purposes. Some rapid tests are currently in use and there is a long list under research or warranting validation and regulatory approval. These kits fall into two categories: the detection of viral components (antigen) or antibodies. Antigen tests detect viral components present during the active infection in the nasopharyngeal specimen (swab). The antibody tests detect the presence of antibodies that appear in the blood following activation of the human immune system in response to the virus (18). Rapid tests are portable, cheaper, easier to use, and take between 10-45 minutes to give a result, compared to molecular PCR tests that take about four hours. However, rapid tests have lower sensitivity and specificity for detection of SARS-CoV-2 virus since infections with other viruses may also give a positive test result, which is not the case for the molecular assays (19, 20).
Treatment for COVID-19: Are we getting closer?
The Food and Drug Administration recently issued a controversial Emergency Use of Authorization (EUA) for two anti-malaria drugs, chloroquine and hydroxychloroquine, based on limited clinical benefit data. On March 20, 2020, a non-randomized clinical trial with the anti-malaria drug hydroxychloroquine in combination with the antibiotic azithromycin gave the world a little hope in fighting COVID-19. Scientists from Marseille, France reported a 100 percent viral clearance in nasopharyngeal swabs in six patients after six days of the combination of hydroxychloroquine and azithromycin (21). But this spark of hope did not last long. Ten days later, another group of scientists from Paris reported no evidence of a strong antiviral activity or clinical benefit of the hydroxychloroquine and azithromycin combination for treatment of their hospitalized patients with severe COVID-19. Eight out of 10 patients still tested positive for SARS-CoV-2 six days after treatment initiation (22). Both these studies were limited by the number of patients enrolled and assessed for short-term benefit only - important aspects when assessing the validity of clinical trial observations. So, currently, the world has no readily available SARS-CoV-2 therapeutics with proven efficacy.
However, scientists around the world are working relentlessly and at an unprecedented pace to develop an ‘effective cure’ for this disease. To date, many potential approaches (summarized in Table 1) are being considered based on the rapid progress of research into SARS-CoV-2. These approaches include: blocking SARS-CoV-2 fusion/entry into human cells, disruption of SARS-CoV-2 replication, suppression of excessive inflammatory response, convalescent plasma treatment and vaccines, as well as the combination of traditional Chinese and Western medicine. Additionally, a number of clinical trials are underway to test the safety and effectiveness of candidate drugs.
Summary
This week, we briefly introduced SARS-CoV-2 and the COVID-19 pandemic and considered the available methods of diagnosis. We also traced the global race against time to identify effective treatments. In our next installment, we will delve into each of these topics in more detail, so stay tuned.
Section Contributors:
Epidemiology: Dr. Yoshie Kobayashi and Dr. Vijay Gupta; Diagnosis: Dr. Salam Salloum-Asfar; Treatment:Dr. Abu Saleh Md Moin; Illustration: Dr. Adviti Naik; and Editors: Dr. Adviti Naik and Dr. Alexandra E. Butler.
For references, please click here
Related News

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention

Huge Potential of HBKU’s Qatar Biomedical Research Institute Convinced Dr. Nasser Hussein Zawia to Accept Opportunity as Research Director

QBRI Insights: Medical Problems and Comorbid Conditions Associated with Autism

Can Gene Signatures Explain Why Some Breast Cancers Are Resistant to Chemotherapy?

QBRI Insights: Extracellular Vesicles as Novel Biomarkers and Therapeutic Targets for Neurological Disorders

FDA-Approved Alzheimer’s Drug Will Help Spur Innovation Toward Personalized Therapies

Alzheimer’s Disease – Warning Signs, Available Treatments and the Guidelines for Prevention